The electron transfer mechanism at electrochemical interfaces is a key theoretical foundation for designing efficient catalytic centers and improving the efficiency of electrical-to-chemical energy conversion. The classical Marcus theory treats the electrolyte as a continuous dielectric medium, suggesting that interactions between reactant molecules, the electrolyte, and the electrode’s catalytic center induce fluctuations in nuclear coordinates, causing interfacial electron transfer to follow the Franck-Condon principle and the law of energy conservation. However, this theory fails to clearly elucidate the orbital characteristics during the electron transfer process. Yan Shicheng’s research group is dedicated to revealing the microscopic orbital characteristics of electron transfer at electrochemical interfaces, providing physical parameters for the design of highly active catalytic centers. In their previous studies on electrochemical oxidation reactions, the group proposed a new mechanism where the empty orbitals of the catalytic center serve as pathways for interfacial charge transfer (Nat. Commun. 2023, 14, 7987; J. Am. Chem. Soc. 2024, 146, 4814). They also developed methods to construct efficient interfacial charge transfer channels by leveraging the thermophysical effects of electrode materials. Examples include thermal suppression of charge disproportionation to stabilize high-valence empty orbital active centers (PNAS 2024, 121, e2316054120), thermal invar effect for modulating spin states (Phys. Rev. Lett. 2024, 133, 258001), and thermal strain regulation of d-band centers (Nat. Commun. 2024, 15, 1780). In their latest study, using the electrochemical nitrate reduction to ammonia as a model reaction system, the group focused on stabilizing the low-spin Co3+(t2g6eg0) catalytic active center through interfacial energetics. The research confirmed that, at the electrochemical reduction reaction interface, electron transfer also follows the empty orbital mechanism. This finding suggests that empty orbitals induced by electrode polarization may serve as a critical indicator for initiating electrochemical reactions.
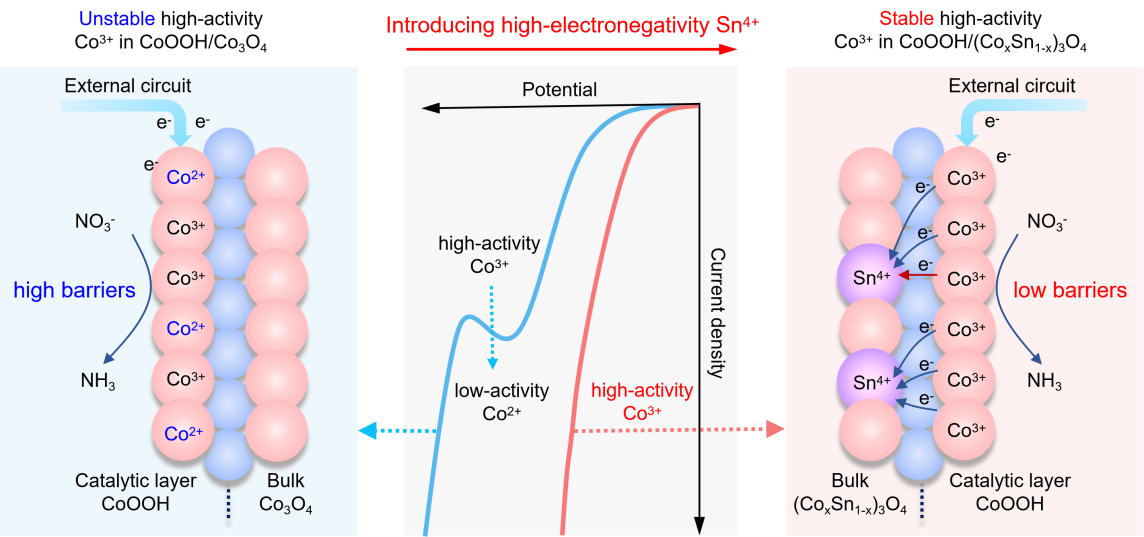
Figure 1. Mechanism schematic of the CoOOH/(CoxSn1-x)3O4 heterojunction for stabilizing Co3+ via the interfacial electric field.
To elucidate the interfacial electron transfer mechanism during the electrochemical nitrate reduction reaction (NO3RR), the research team designed a CoOOH/(CoxSn1-x)3O4 heterojunction catalyst. The principle lies in: by incorporating tin (Sn) into the Co3O4 substrate to form CoOOH/(CoxSn1-x)3O4, the work function difference between it and the CoOOH catalytic layer is increased, thereby significantly enhancing the heterojunction interfacial electric field. This strengthened interfacial electric field, under negative voltage (reduction potential), stabilizes the low-spin Co3+(t2g6eg0) active centers in the CoOOH layer. These stable and efficient catalytic active centers enable persistent and highly efficient NO3RR catalysis at high current densities, thereby facilitating in-depth studies on the electron transfer mechanism.
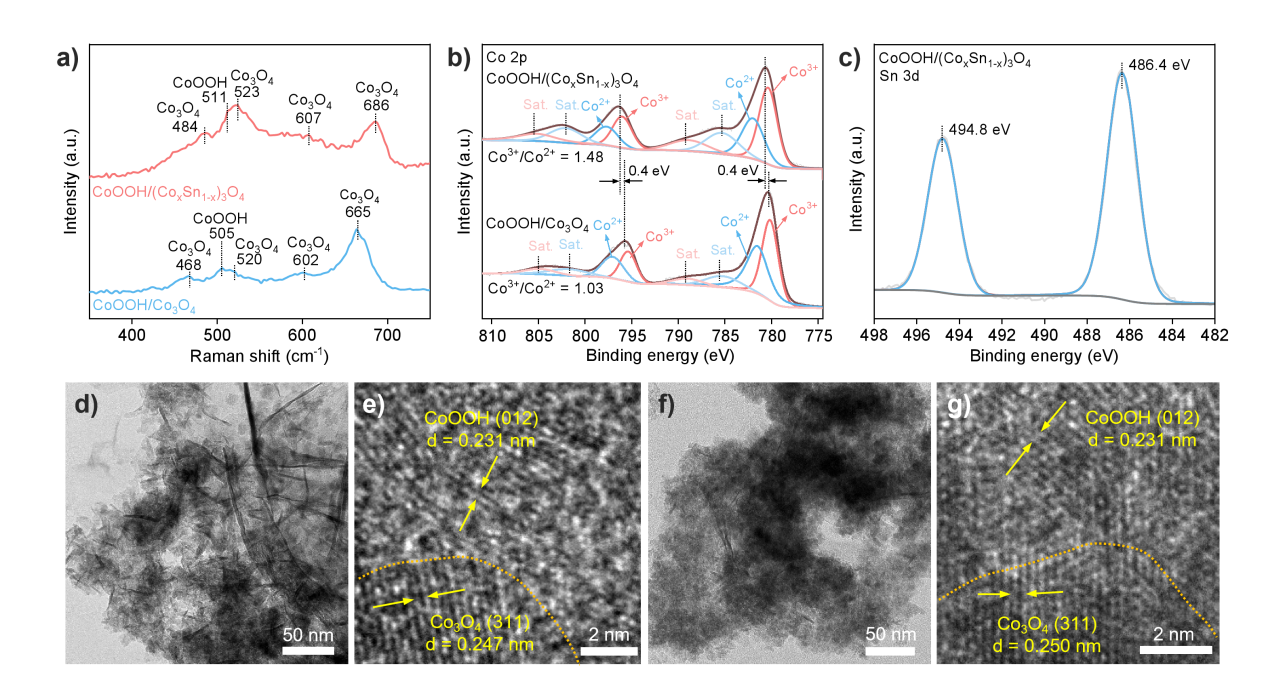
Figure 2. Structural characterization of CoOOH/Co3O4 and CoOOH/(CoxSn1-x)3O4. a) Raman spectra; b) Co 2p XPS spectra; c) Sn 3d XPS spectra; d)-e) HRTEM images of CoOOH/Co3O4; f)-g) HRTEM images of CoOOH/(Cox Sn1-x)3O4.
The CoOOH/Co3O4 and CoOOH/(CoxSn1-x)3O4 catalysts were prepared using the electrochemical deposition method. Raman spectroscopy characterization confirmed the coexistence of spinel-type Co3O4 and CoOOH in the catalysts. XPS fitting analysis showed that, compared to the undoped CoOOH/Co3O4, the Co3+/Co2+ ratio in the Sn-doped CoOOH/(CoxSn1-x)3O4 was significantly increased. This phenomenon is attributed to the higher electronegativity of Sn4+ (1.706) than that of Co3+ (1.693), indicating that Sn doping helps stabilize the high-valence state of Co (Co3+). High-resolution transmission electron microscopy (HRTEM) observations further revealed that after Sn doping, the (311) interplanar spacing of (CoxSn1-x)3O4 increased from 0.247 nm for the undoped Co3O4 to 0.250 nm. This lattice expansion effect mainly originates from the larger ionic radius of Sn4+ (0.069 nm) compared to that of Co3+ (0.061 nm).

Figure 3. Electrocatalytic NO3RR performance characterization of CoOOH/Co3O4 and CoOOH/(CoxSn1-x)3O4. a) LSV curves; b) I-t fitted LSV curves; c)-d) Faradaic efficiency of NH4+ and NO2-; e) Comparison of NH4+ Faradaic efficiency tested by different methods; f) NH4+ yield; g) Cycling stability test; h) Chronopotentiometry stability test.
At a potential of 0.1 V (vs. RHE), both CoOOH/Co3O4and CoOOH/(CoxSn1-x)3O4 catalysts exhibit initial activity for the nitrate reduction reaction. However, CoOOH/(CoxSn1-x)3O4 demonstrates superior intrinsic NO3RR activity and faster reaction kinetics. In contrast, CoOOH/Co3O4 shows a significant reduction peak during the reaction, which is attributed to the reduction of highly active Co3+ to less active Co2+. This reduction process significantly increases the overpotential of the reaction. The CoOOH/(CoxSn1-x)3O4 catalyst achieves a Faradaic efficiency of 96.7% for NH4+ at -0.3 V (vs. RHE). Furthermore, the catalyst exhibits no performance degradation after 10 consecutive cycling tests and 1000 hours of continuous operation at a constant current density of -100 mA/cm2. In summary, the CoOOH/(CoxSn1-x)3O4 catalyst demonstrates outstanding catalytic activity, high selectivity, and excellent long-term stability in the nitrate reduction reaction.
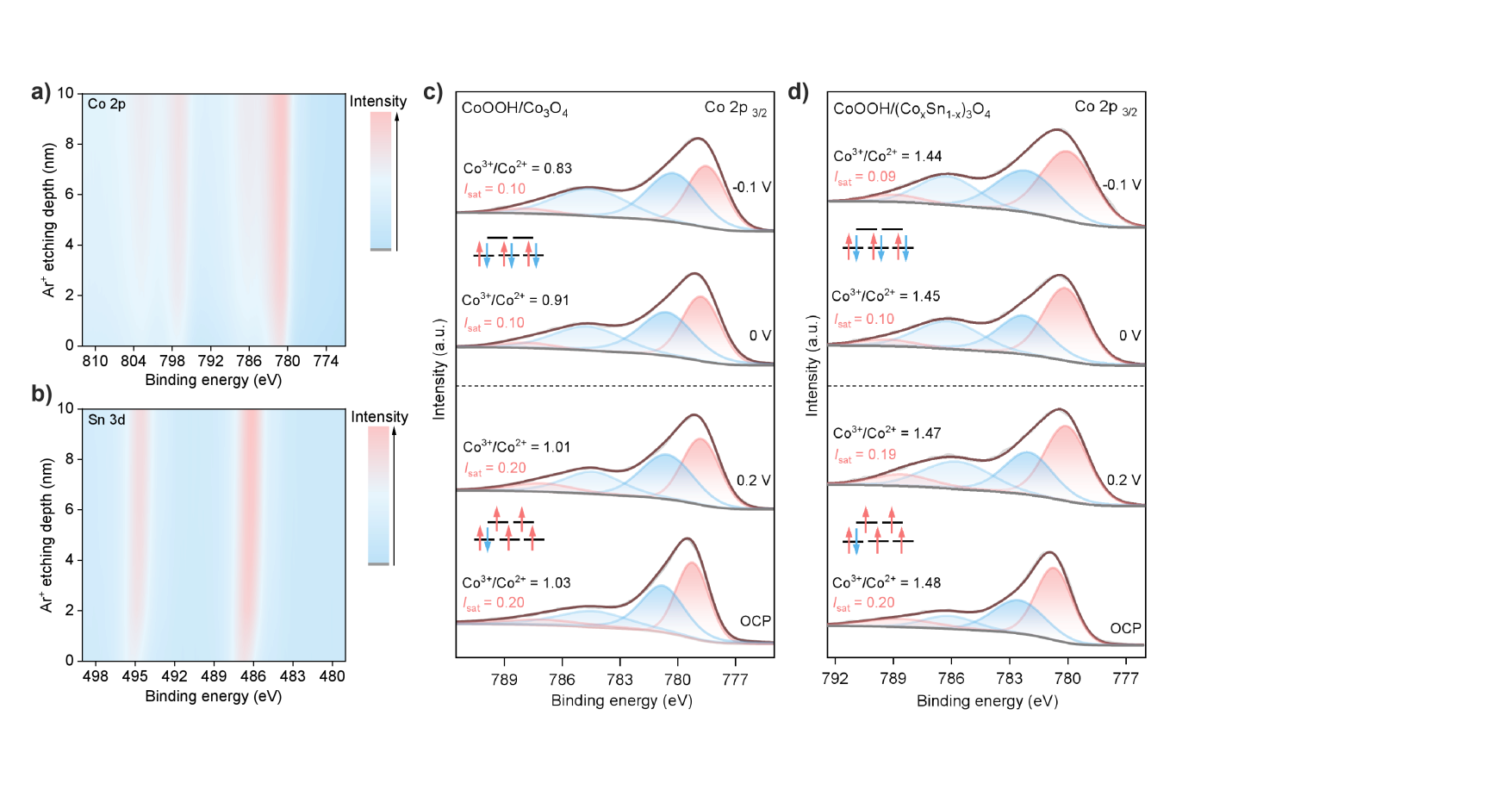
Figure 4. Spin state transition and stability of active centers. a) Co 2p and b) Sn 3d XPS depth profiling of CoOOH/CoOOH/(CoxSn1-x)3O4; c) In situ XPS spectra of the Co 2p3/2 core level for CoOOH/Co3O4 and d) CoOOH/(CoxSn1-x)3O4.
XPS depth profiling results show that within the 0-10 nm depth range, the contents of Co and Sn remain stable, confirming the successful doping of Sn and its uniform distribution. In situ XPS further characterized the changes in valence and spin states during the electrochemical reaction: the satellite peak intensity (Isat) is defined as Isat = Asat/(Asat + Aprimary), where Asat and Aprimary are the peak areas of the satellite peak and the main Co 2p3/2 peak after spectral fitting, respectively. Isat is positively correlated with the spin state: a higher Isat corresponds to a higher spin state. Furthermore, the characteristic satellite peak positions can distinguish between Co2+ and Co3+ species: Co2+ exhibits a strong satellite peak at 4-6 eV above the main peak, while Co3+ shows a weak satellite peak at 9-10 eV higher binding energy. Analysis of Co 2p spectra at open circuit potential indicates that both CoOOH/Co3O4 and CoOOH/(CoxSn1-x)3O4 are in a high spin state, with consistent Isat values (0.2). However, there is a significant difference in the oxidation state: the Sn-doped material has a higher Co3+/Co2+ ratio (1.48, compared to 1.03 for the undoped material), indicating that Sn doping stabilizes Co3+ species. Adjusting the potential from 0.2 V to -0.1 V induced significant changes: in CoOOH/Co3O4, the Co3+/Co2+ ratio decreased significantly from 1.03 to 0.83, indicating that Co3+ is reduced to less active Co2+ under cathodic conditions; whereas in CoOOH/(CoxSn1-x)3O4, the Co3+/Co2+ ratio remained stable (only slightly changing from 1.48 to 1.44), confirming that Sn doping effectively protects the active Co3+ centers from reduction. Simultaneously, when the potential is below 0 V, the Isat values of both catalysts decreased from 0.2 to 0.1 (at 0 V), indicating that Co3+ transitions from a high spin state (t2g4eg2, with singly occupied eg orbitals) to a low spin state (t2g6eg0, with unoccupied eg orbitals). This spin state transition is crucial as it provides the unoccupied orbitals required for electron transfer, thereby initiating NO3RR.
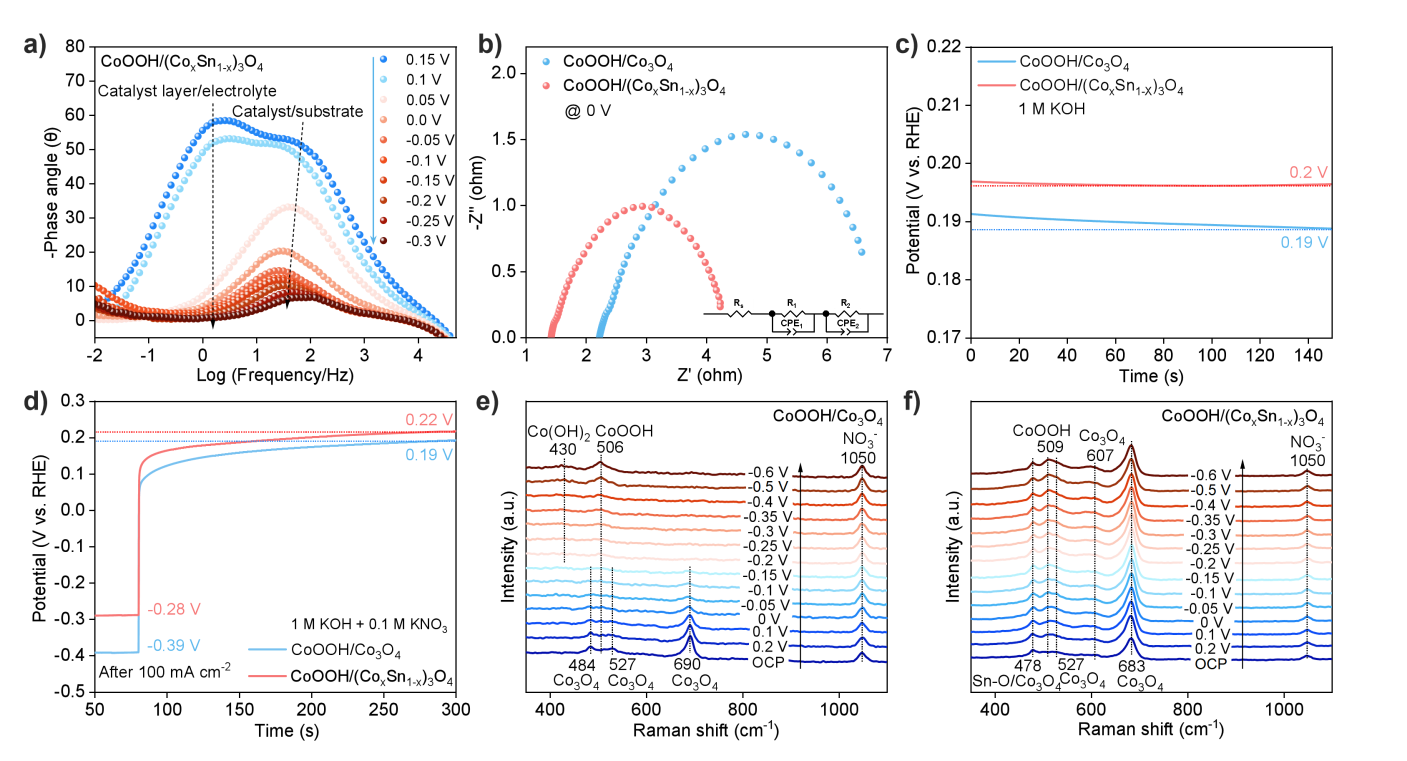
Figure 5. Electron Transfer and Identification of Active Centers. a) In situ EIS Bode plot of CoOOH/(CoxSn1-x)3O4; b) EIS Nyquist plot at 0 V; c) Open circuit voltage test; d) Open circuit voltage test after polarization at a current density of 100 mA/cm2; e) In situ Raman spectra of CoOOH/Co3O4 and f) CoOOH/(CoxSn1-x)3O4.
Electrochemical impedance spectroscopy analysis reveals that Sn doping significantly reduces the interfacial resistance of CoOOH/(CoxSn1-x)3O4. Meanwhile, this system exhibits a higher open-circuit voltage, indicating a stronger interfacial built-in electric field. Furthermore, after polarization treatment at -100 mA/cm2, CoOOH/(CoxSn1-x)3O4 maintains a relatively high open-circuit voltage, suggesting a higher average oxidation state of its surface-active catalytic species. In situ Raman spectroscopy results confirm that CoOOH is the primary active phase during the reaction. For the undoped CoOOH/Co3O4, some Co3+ species are reduced to Co(OH)2 under negative potentials. In contrast, for CoOOH/(CoxSn1-x)3O4, the Co3+ active sites exhibit enhanced stability due to the strong interfacial interactions of the heterojunction, which directly contributes to improved catalytic activity and stability.
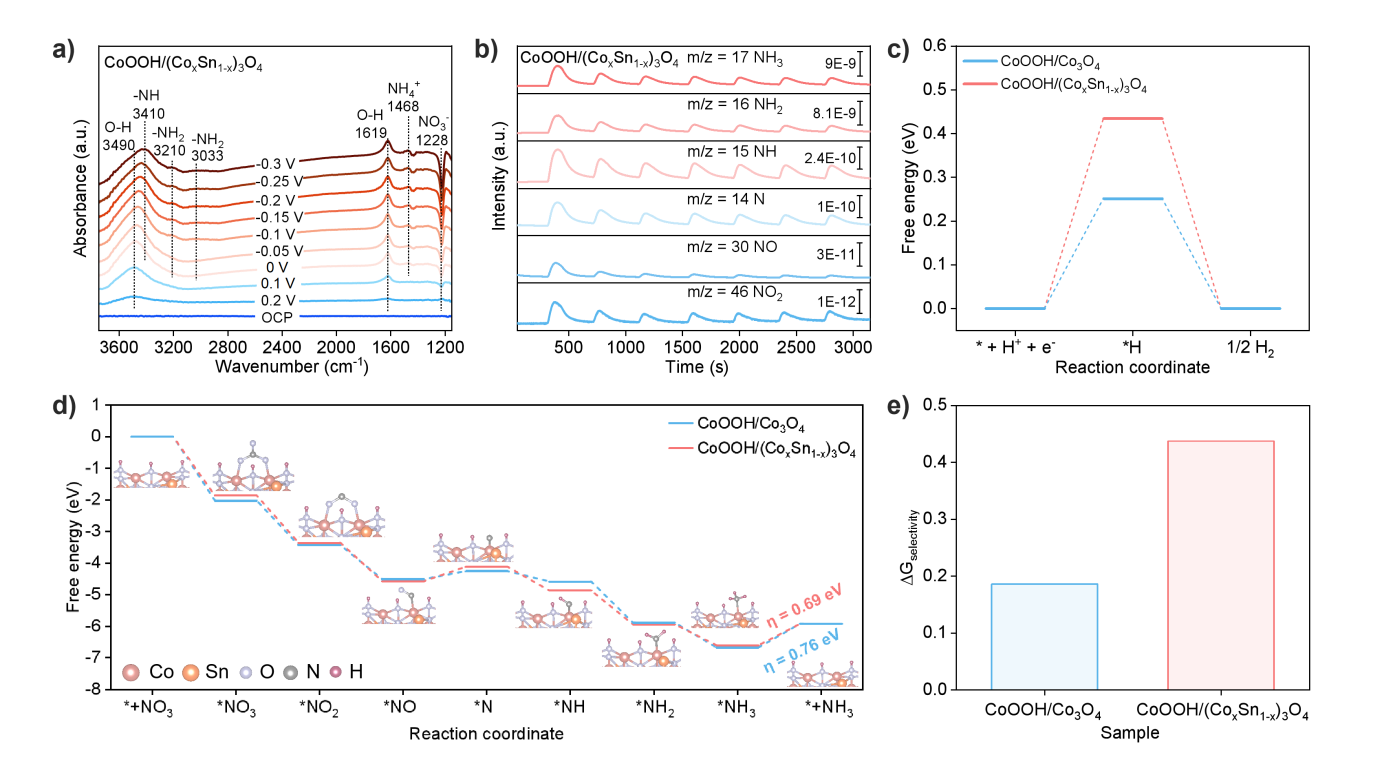
Figure 6. Electrochemical reaction mechanisms of CoOOH/Co3O4 and CoOOH/(CoxSn1-x)3O4. a) In situ electrochemical ATR-SEIRAS spectra; b) Online electrochemical mass spectrometry; c) Reaction free energy of hydrogen evolution; d) Free energy of nitrate reduction reaction; e) Gibbs free energy of nitrate reduction reaction product selectivity.
By combining in situ electrochemical ATR-SEIRAS with online electrochemical mass spectrometry, we have proposed the reaction pathway for NO3RR: NO3- → *NO3 → *NO2 → *NO → *N → *NH → *NH2 → *NH3 → NH3. Further density functional theory calculations of the Gibbs free energy for adsorbed intermediates revealed that the CoOOH/(CoxSn1-x)3O4 catalyst offers the following advantages: a lower energy barrier for the rate-determining step (i.e., NH3desorption), indicating more favorable kinetics; a higher Gibbs free energy for hydrogen adsorption (which suppresses the competing hydrogen evolution reaction); and higher reaction selectivity toward NO3RR. These results collectively confirm the excellent selectivity of CoOOH/(CoxSn1-x)3O4 for NO3RR.
This study reveals that empty orbitals, serving as key electron transfer channels, play a central role in regulating the initiation and efficiency of electrochemical reactions. Specifically, the initiation of the reaction depends on the establishment of electron transfer pathways through empty orbitals under applied voltage. For electrochemical reduction reactions, the spin-state transition of the catalytic center is an effective way to create empty orbitals. This finding provides an orbital-level theoretical basis for the design of highly active catalytic centers: regulating the transition of electronic spin configurations is an important approach to forming empty orbitals in catalytic centers, while constructing interfacial electric fields can stabilize these empty orbitals under high current densities. The related research findings have been published in Angewandte Chemie International Edition under the title "Unlocking Durable and Efficient Nitrate-to-Ammonia Electrocatalysis via Interface-Stabilized Trivalent Cobalt" (doi: 10.1002/anie.202522042). Qian Zheng (2022 Ph.D. candidate), Zehua Liu (2024 Master’s candidate), and Yuandong Yan (2022 Ph.D. candidate) from the College of Engineering and Applied Sciences at Nanjing University are co-first authors of the paper, with Professor Shicheng Yan serving as the corresponding author. The research was conducted under the guidance of Academician Zhigang Zou and received support from the National Natural Science Foundation of China and the Scientific and Technological Innovation Project of Carbon Emission Peak and Carbon Neutrality of Jiangsu Province.
Keywords:Electrochemical interfacial electron transfer mechanism; Empty orbitals; Electrochemical reduction reaction; Efficient catalytic center design
Paper Information:
Title: Unlocking Durable and Efficient Nitrate-to-Ammonia Electrocatalysis via Interface-Stabilized Trivalent Cobalt
Authors: Qian Zheng, Zehua Liu, Yuandong Yan, Shicheng Yan*, and Zhigang Zou
Link: https://doi.org/10.1002/anie.202522042

